Efficacy and Safety of The Herbal Combination Containing Tropaeoli Majoris Herba and Armoraciae Rusticanae Radix in Patients Suffering from Uncomplicated, Acute Rhinosinusitis: A Randomized, Double-Blind, Placebo Controlled, Two-Arm, Parallel Group, Phase
Abstract
Objectives: Demonstration of the efficacy and safety of an herbal combination containing horseradish root and nasturtium herb for the treatment of uncomplicated, acute rhinosinusitis.
Design: Randomized, double-blind, placebo-controlled, two-arm, parallel group, multicenter, phase IV clinical study.
Setting: Sixteen centers in Germany, from October 2017 to March 2018.
Participants: Adult patients (≥18 – 75 years, male and female) diagnosed with uncomplicated, acute rhinosinusitis.
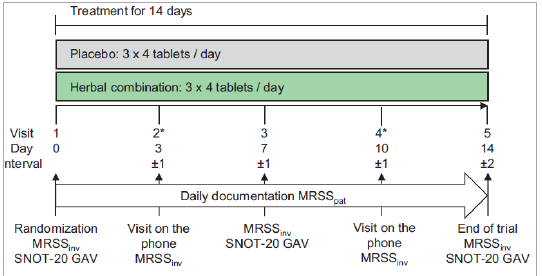
Interventions: Participants received the herbal combination or placebo (3 x 4 per day) for 14 days.
Clinical trial registration: EudraCT 2017-002081-40.
Endpoints: Primary endpoint was MRSSinv/MRSSpat documented between day 6 and day 10, computed as AUC, assessed by ANCOVA with day 3 as covariate. MRSSinv/MRSSpat, responder rates, efficacy assessment and SNOT-20 GAV were used as secondary endpoints.
Results: Out of 380 randomized participants, 238 were included in the FAS for statistical analysis. Treatment with the herbal combination revealed a significant smaller AUC (14.99) compared to placebo (18.52, p=0.0003). Moreover, MRSSinv/MRSSpat was significantly lower after administration of the herbal combination in comparison to placebo (3.60 herbal combination vs. 4.40 placebo, p=0.0018). Responder rates were significantly higher at visit 3 of patients receiving the herbal combination compared to placebo (92.1% herbal combination vs. 83.3% placebo, p=0.0418). Adverse events occurred in 21.9% and 18.6% of participants receiving the herbal combination and placebo, respectively. Most common adverse events were headaches and gastrointestinal complaints.
Conclusion: Efficacy and safety of the herbal combination in comparison to placebo were confirmed while the treatment was well tolerated.